Fisher & Paykel Healthcare unveils new Airvo™ 3 high flow system
Fisher & Paykel Healthcare Corporation Limited announced today it has released the Airvo 3 high flow system.
The new respiratory device builds upon the market-leading Airvo 2, offering more advanced technology and a broader range of features.
“We are excited to bring the Airvo 3 to market following more than five years of research and development,” said Lewis Gradon, Managing Director and Chief Executive Officer at Fisher & Paykel Healthcare. “The new device will more easily allow for the treatment of patients as they move through different parts of the hospital.”
Designed to deliver Optiflow™ therapy, the new device incorporates the company’s OptiO2™ closed-loop system for targeted oxygen delivery, which helps to ensure the patient is provided the right level of oxygen. Other new features include an integrated battery, a large touchscreen interface, expanded settings for pediatric and neonatal patients, and an increased maximum flow rate of 70 litres per minute compared to 60 litres per minute on the Airvo 2.
“Our teams worked closely with our customers and clinicians to design a device that meets healthcare providers’ evolving needs and deliver better patient outcomes,” said Andrew Somervell, VP – Products and Technology for Fisher & Paykel Healthcare. “We’re pleased to now see the Airvo 3 in New Zealand hospitals and we look forward to it being more widely available in due course.”
“The new product reflects our continued focus on our longer-term aspirations despite the challenges of responding to demand during the pandemic,” said Mr Gradon. “We did not let this once-in-generation event divert us from our long-term course. Our teams have continued to innovate across our product groups – the Airvo 3 is one such example.”
A controlled market release of the Airvo 3 is now underway in New Zealand and it will be available in more markets as regulatory clearances are received.
Airvo 3 Images:
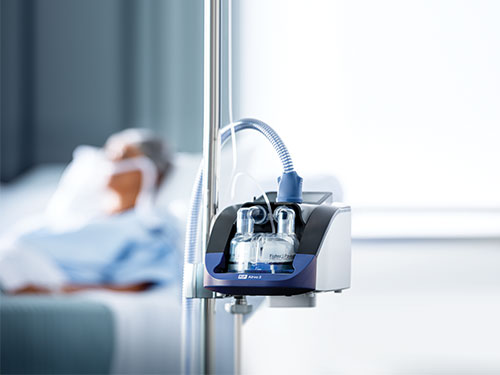
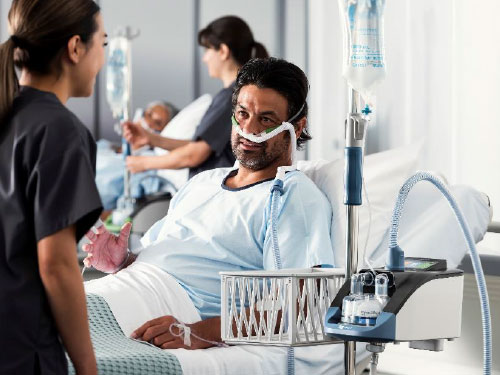
About Fisher & Paykel Healthcare
Fisher & Paykel Fisher Healthcare is a leading designer, manufacturer and marketer of products and systems for use in acute and chronic respiratory care, surgery and the treatment of obstructive sleep apnea. The company’s products are sold in more than 120 countries worldwide. For more information about the company, visit our website www.fphcare.com.
Ends
Contact
|