View key clinical references:
What is invasive ventilation?
Invasive ventilation, including conventional mechanical ventilation and high-frequency oscillatory ventilation, refers to respiratory support delivered directly to a patient’s lower airways via an endotracheal (ET) or tracheostomy tube. These modes of delivery:
- bypass the natural mechanisms of filtration, humidification and warming, which are typically provided by the upper airways
- inhibit primary mechanisms of airway defense such as coughing, sneezing, gagging and particle filtration.
A ventilator is required for invasive respiratory support to enable – or support – lung function and gas exchange.
View these resources for more information on:
Negative and positive pressure ventilation (article by John Landry, BS, RRT)
Volume and pressure controlled ventilation (YouTube video by Respiratory Therapy Zone)
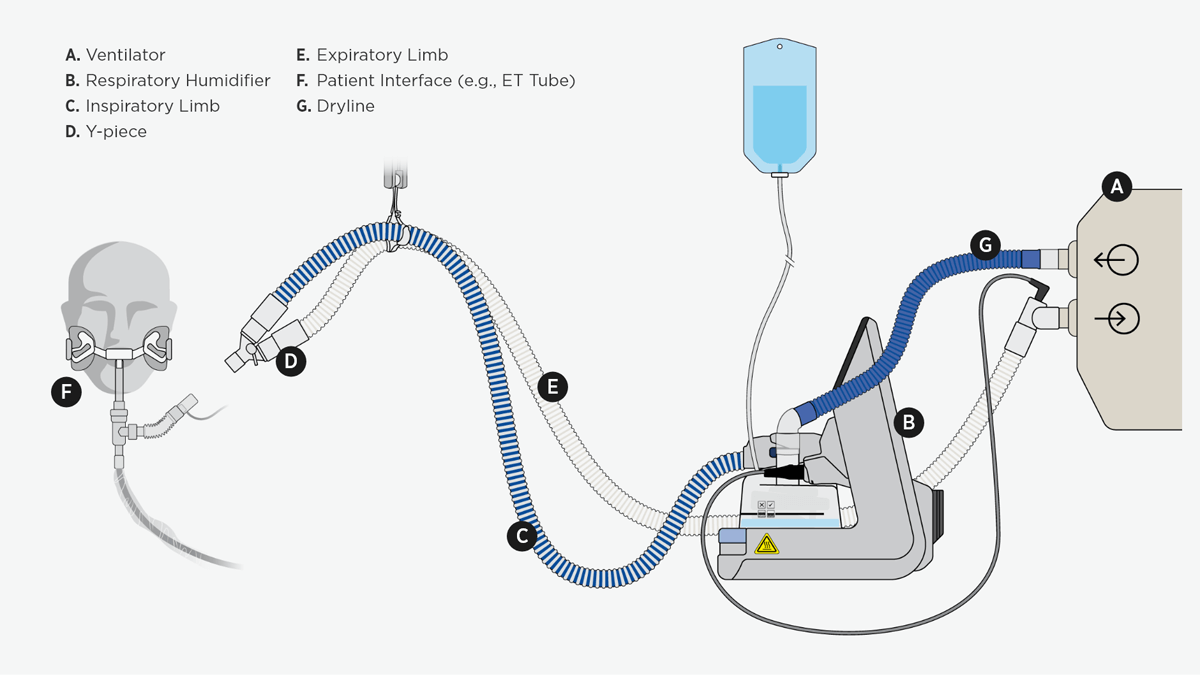
This diagram shows an invasive ventilation system incorporating a heated humidifier that warms and moistens the air.
Why is humidity important for invasive ventilation?
The heating and humidifying of respiratory gases is crucial and mandated in clinical guidelines for invasively ventilated patients.
When the primary mechanisms of the upper airway are bypassed with the presence of a tracheostomy or ET tube in invasively ventilated patients, the mucociliary transport system becomes the last line of airway defense against debris and other pathogens. This system relies strongly on the heat and humidity of inspired air to function optimally.
Delivering gases at or as close as possible to 37 °C and fully saturated (44 mg/L H2O) helps restore and support natural mucociliary clearance that aids the transportation of debris up and out of the airway.1 In addition, it allows the patient to conserve energy as the airway doesn’t need to provide additional heat or moisture to the inspired gases.
Learn about principles of heat and humidity in the airway
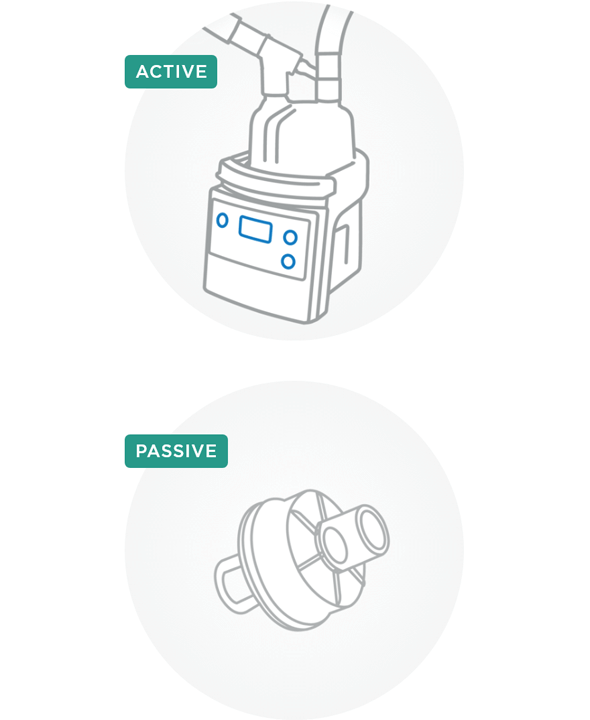
How can humidity be added to inspired gas?
There are two types of humidification devices that can add heat and humidity to inspired gas – active and passive.
Active humidification devices utilize an external water source to add humidity to the inspired air. While these devices can be unheated, such as in-line vaporizers or bubble humidifiers, heated humidifiers (HH), including passover and counterflow humidifiers, are commonly used. Unlike unheated humidifiers, HHs use an additional heat source to condition the inspired gas. The suggested levels of humidity for active humidifiers are between 33 and 44 mg/L H2O and temperatures range from 34 to 41 °C.2
Passive humidification devices, including heat and moisture exchangers (HMEs), utilize the heat and moisture in patient-expired air to condition the next breath. HMEs can be hygroscopic, hydrophobic or hydrophobic-hygroscopic and are placed between the Y-piece of the ventilator circuit and the patient interface. The efficiency of passive devices varies with design and can have implications for ventilation3 such as increased dead space and expiratory resistance.
Why choose heated humidification over HMEs?
Improved humidity
Because heated humidifiers are independent of a patient’s respiratory function, they minimize heat and water loss compared with passive methods that rely on patient-expired heat and humidity for inspired gas conditioning. 4
A large body of clinical evidence demonstrates the effect of heated humidification on mucociliary function and clearance, including a reduction in ET-tube-obstruction rates, compared with passive humidification.5-11
Learn about heated respiratory humidification
Improved ventilation
Lung-protective ventilation (LPV) refers to mechanical ventilation strategies that use tidal volumes and pressures closer to physiological values than conventional mechanical ventilation settings. These strategies aim to prevent ventilator-associated lung injury and are largely considered standard practice for invasive ventilation.12,13
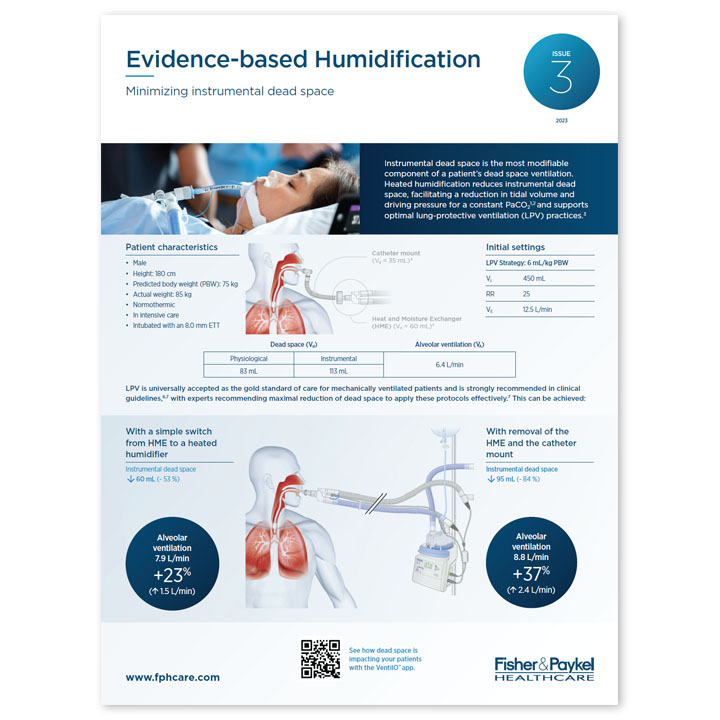
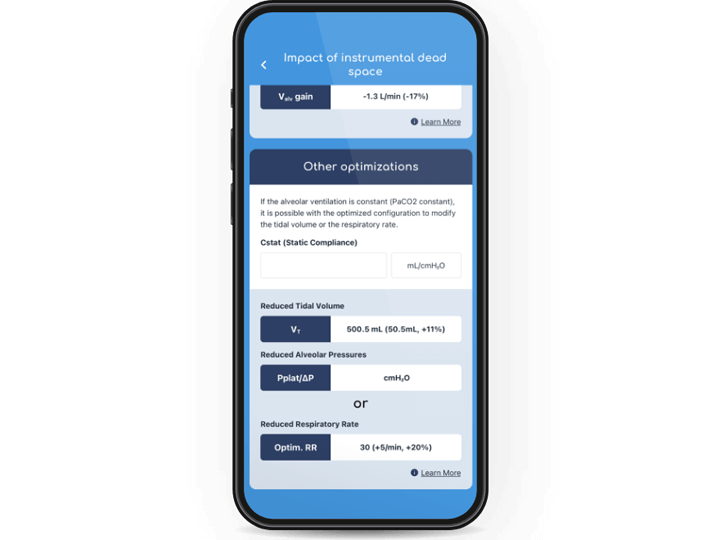
Instrumental dead space is particularly crucial when considering effective application of LPV techniques. It refers to the volume of ventilated air that does not participate in gas exchange, caused by any additional equipment after the Y-piece of the ventilator circuit. Increases in instrumental dead space can have a negative effect on a patient’s ability to clear CO2 from their airways.
Unlike HMEs, HHs don’t contribute to additional instrumental dead space. The body of literature has shown that using heated humidification rather than passive devices allows for the effective implementation of LPV, facilitating reductions in tidal volumes and PaCO214,15 without affecting cerebral perfusion.16 The use of active humidification has also been shown to reduce inspiratory effort and the work of breathing.14,17

Working alongside the continuum of care.
Fisher & Paykel Healthcare's world-leading respiratory devices work alongside the continuum of care to support patients of all ages throughout the hospital.
This product is not available for purchase by the general public. Always follow the directions for use.
The VentilO App is not intended to provide medical diagnoses or treatment advice. The app is intended exclusively for healthcare professionals and is not a substitute for the exercise of clinical judgement by an appropriately qualified healthcare professional.
VentilO is a trademark of L’Institut universitaire de cardiologie et de pneumologie de Québec.
Apple and the Apple logo are trademarks of Apple Inc., registered in the U.S. and other countries. The App Store is a service mark of Apple Inc., in the U.S. and other countries. Google and Google Play are trademarks of Google LLC.