Humidity refers to water vapor (water in its gaseous form) suspended within a gas. A water-vapor molecule is much smaller than common bacteria and viruses, so cannot carry these pathogens.
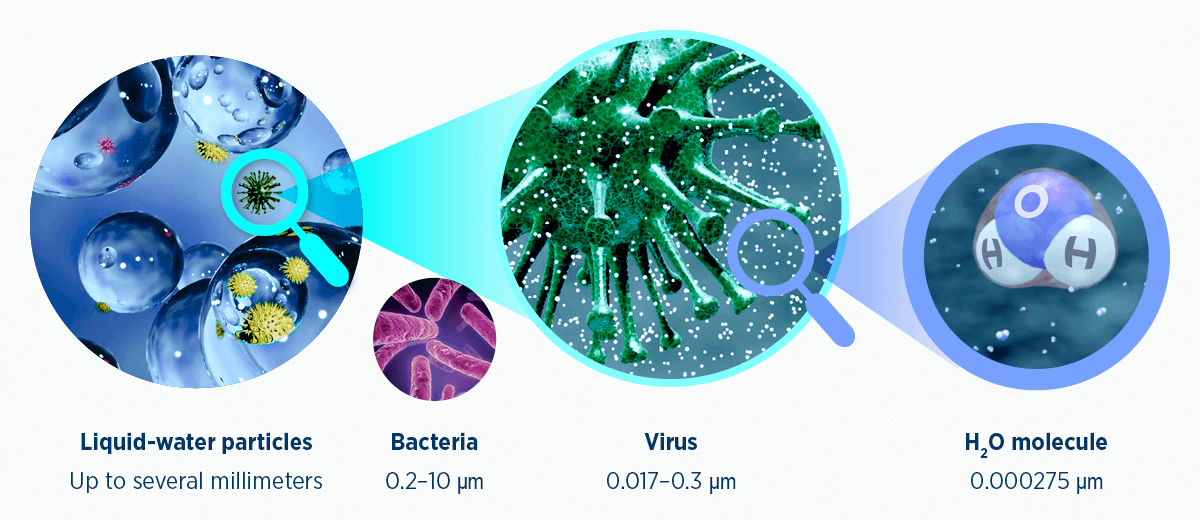
Figure 1. Relative sizing of common pathogens to water in both liquid and gas states. μm, micrometers (microns); 1 mm = 1000μm
There are three key concepts commonly used when discussing humidity:
Absolute humidity
Absolute humidity (AH) is the physical amount of water vapor present per liter of gas, measured as mg/L H2O. This refers to the water content alone – it has no relation to gas temperature.
Relative humidity
Relative humidity (RH) refers to the amount of water vapor per liter of gas, compared with the maximum possible amount the gas could hold. This means it’s always expressed as a percentage value.
The capacity of a gas, and therefore the RH, is dependent on temperature. Warmer gases can hold more water vapor.
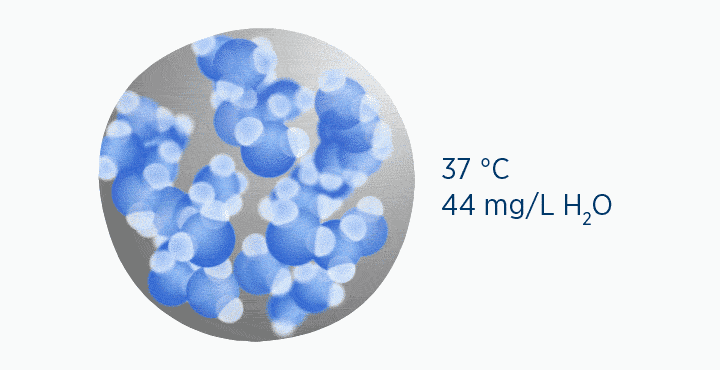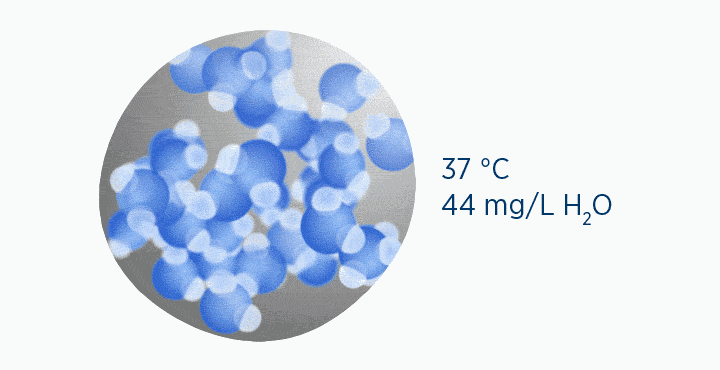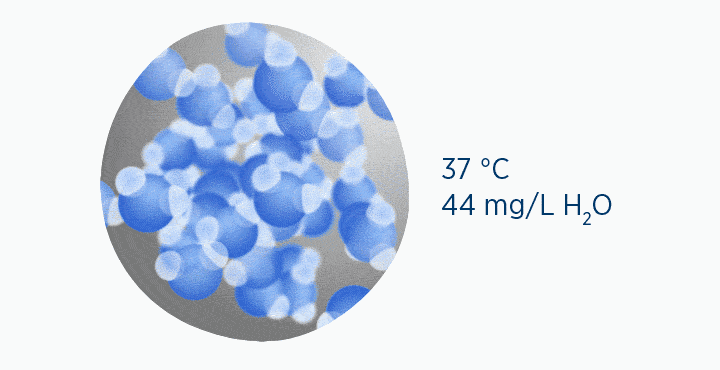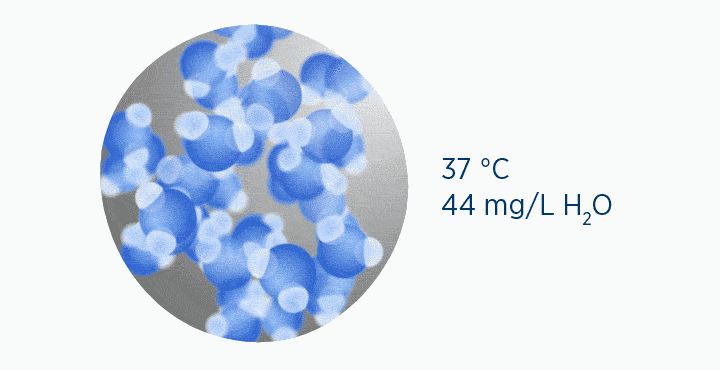
In this animation, the grey circle represents the capacity of the gas, while the dark blue circle represents how much water vapor is in the gas.
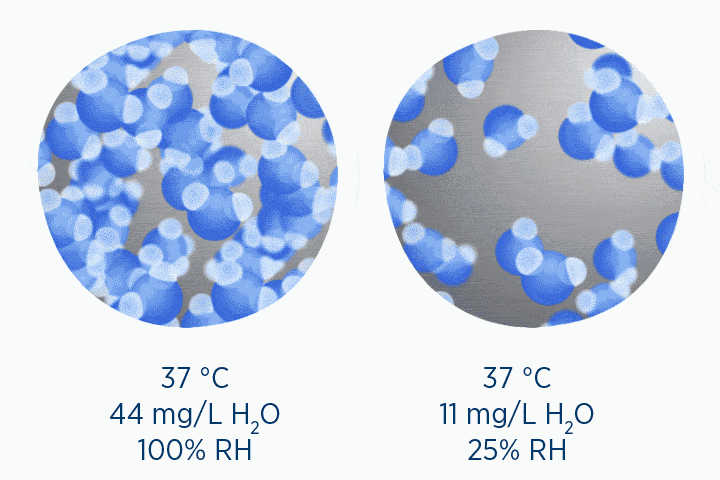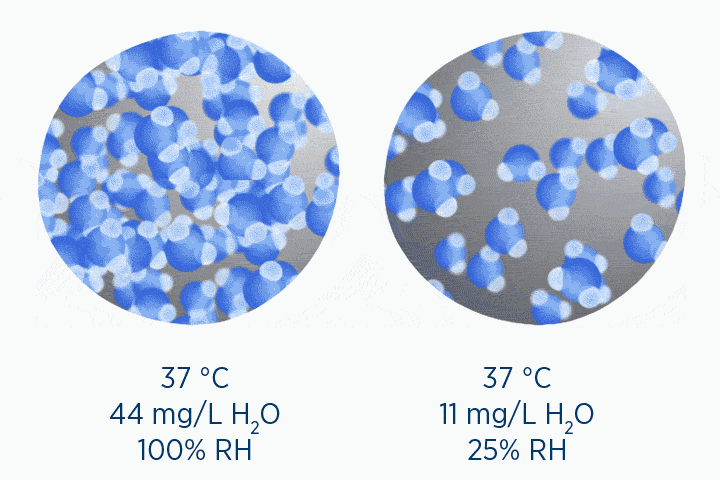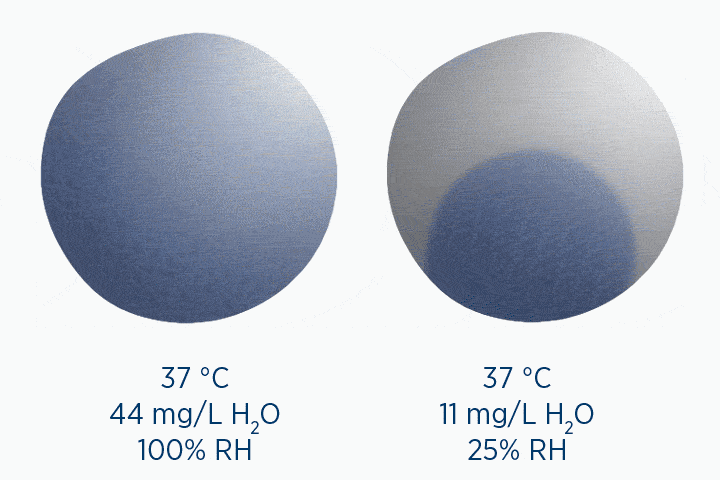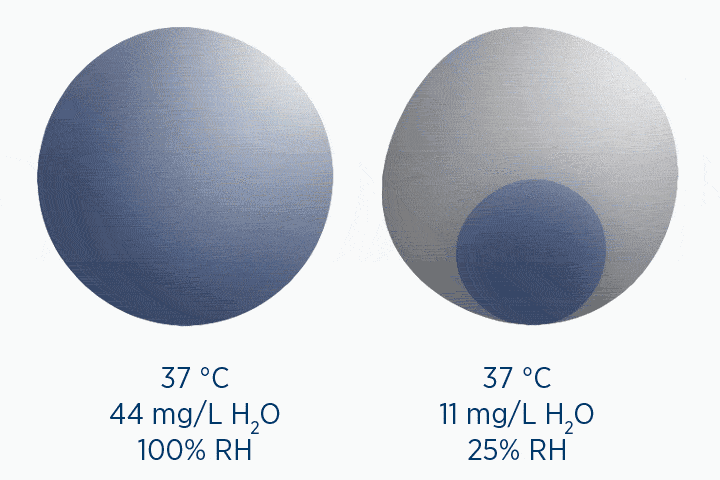
When at 100% RH, holding 44 mg/L H2O of absolute humidity – the gas is saturated and cannot hold additional water vapor.
In the second image, the gas is the same temperature, so the grey circle is the same size (it can still only hold 44 mg/L H2O of water vapor). However, the gas only contains 11 mg/L H2O (the blue circle), correlating to a RH of 25%.
Dew point
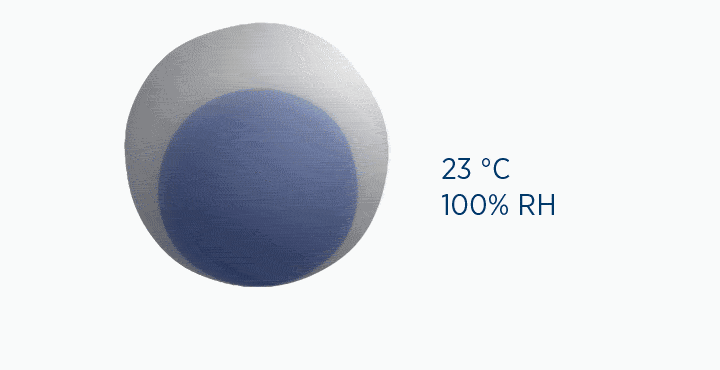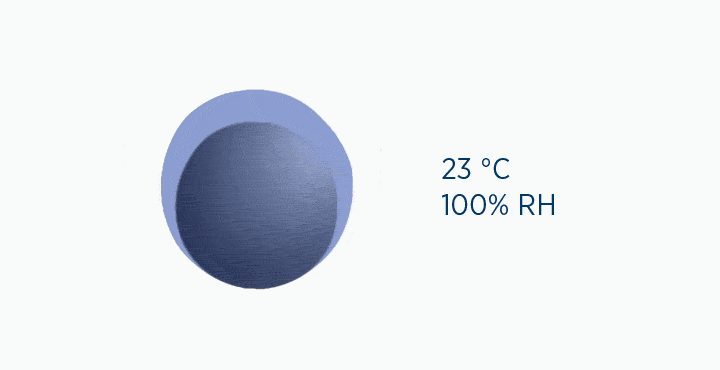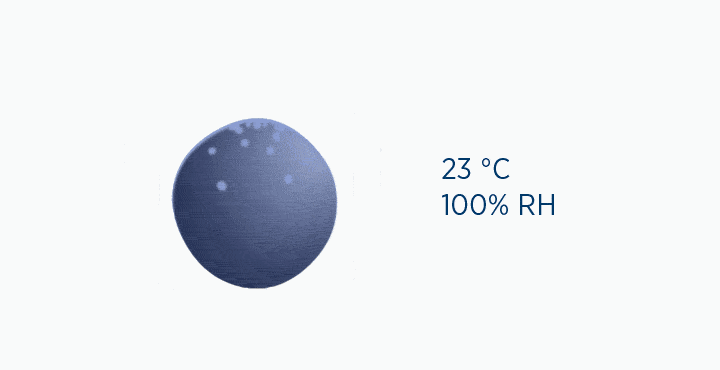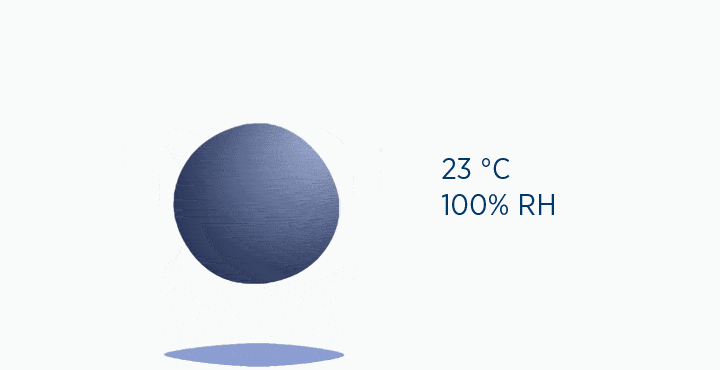
Dew point is the temperature at which the gas is saturated – that is, when it is holding the maximum amount of vapor possible.
If the temperature falls below this point, the capacity of the air is reduced, and the excess water vapor must be lost as condensation.
In the dew point animation, the gas is initially at 37 °C, holding 44 mg/L H2O. However, the gas then cools to 23 °C. This reduces its maximum capacity from 44 to 21 mg/L H2O. The extra water vapor it can no longer hold is lost as liquid water, through the process of condensation.
Heating and humidifying gas uses energy.
- Sensible heat refers to the amount of energy used to change the temperature of the gas.
- Latent heat of vaporization is the amount of energy required to convert water into water vapor, increasing the gas’ absolute humidity.
It’s important to know that humidifying gas uses much more energy than heating it. Eighty-five per cent of the energy we use conditioning air is used to increase water vapor content.
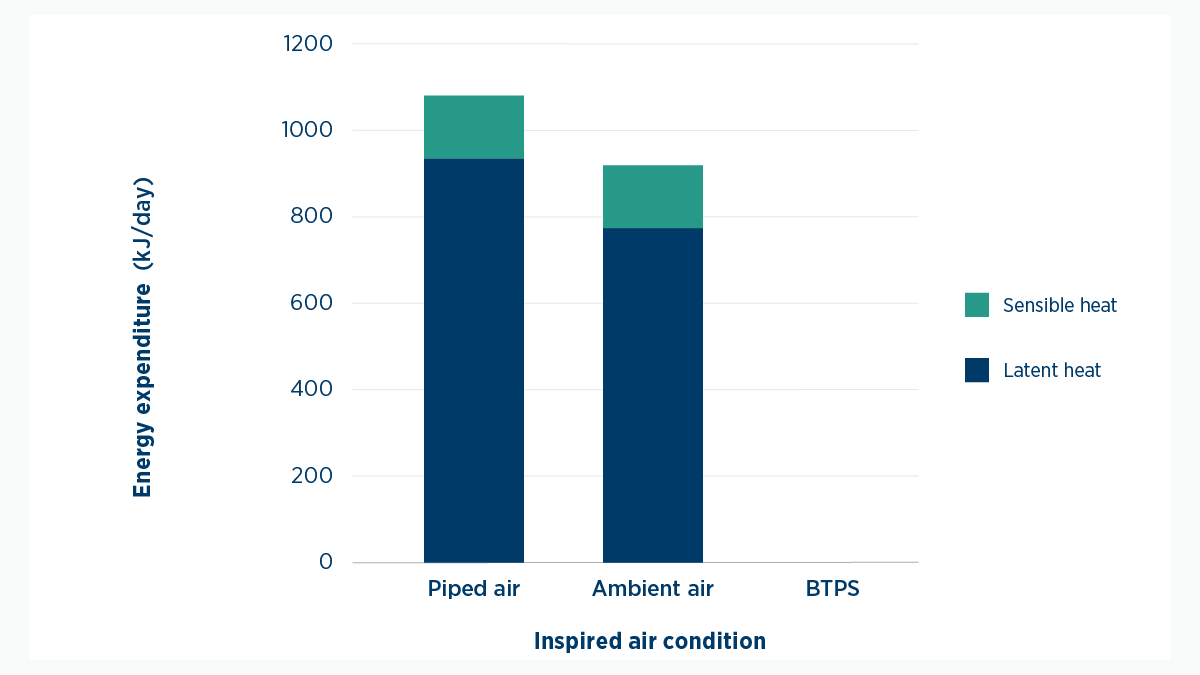
Figure 5. Energy expenditure required to condition gases relative to inspired air conditions. Values calculated represent an adult with a tidal volume of 500 mL, and a respiratory rate of 12 breaths per minute.
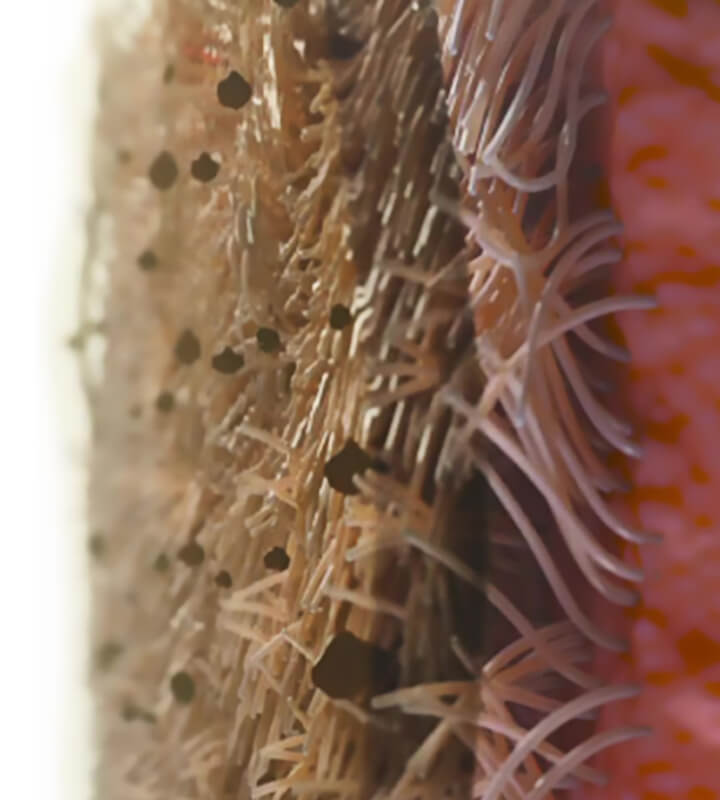
Heat and Humidity in our Airways
For our airways to heat and humidify the air we inspire, it needs to pull heat and water from a source.
The mucociliary transport system (MTS) is a crucial piece of airway physiology, and one of the core contributors to heating and humidifying air.1 It refers to the coordinated action of three layers that line the airway.
- A mucus layer.
- An aqueous (sol) layer.
- A ciliated epithelial layer.
Together, these layers form the MTS and help protect the lungs. The mucus is sticky, and traps inhaled debris. The cilia reach through the aqueous layer to push the mucus upward where it is swallowed or coughed out of the airway.
The effectiveness of this system is strongly dependent on the heat and humidity of inspired air.2 Colder, drier air impacts the viscosity of the mucus, the depth of the aqueous layer, and both the coordination of the cilia and frequency at which they beat.
Heated, humidified gas helps airway secretions remain fluid, and supports continued mucociliary clearance.1–4