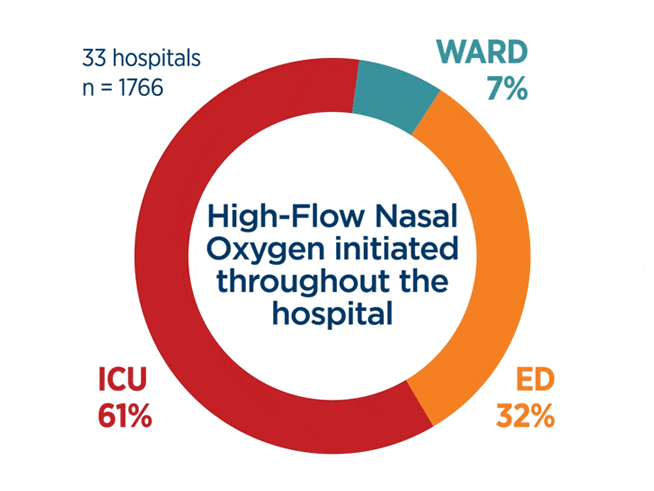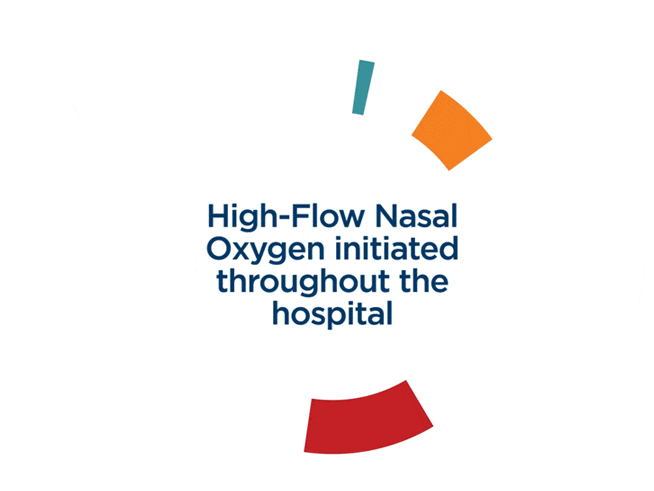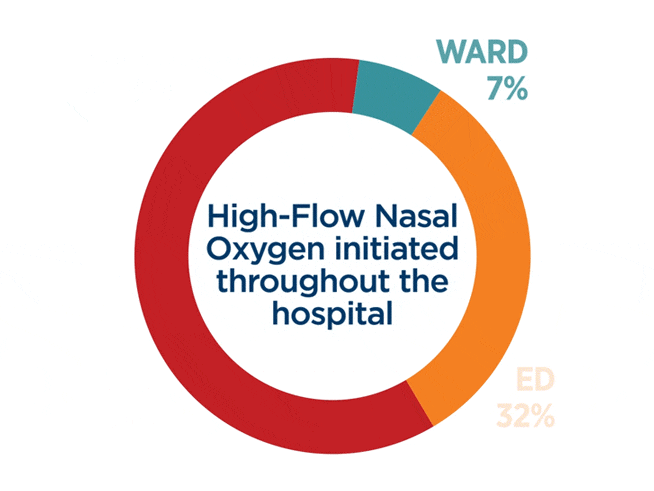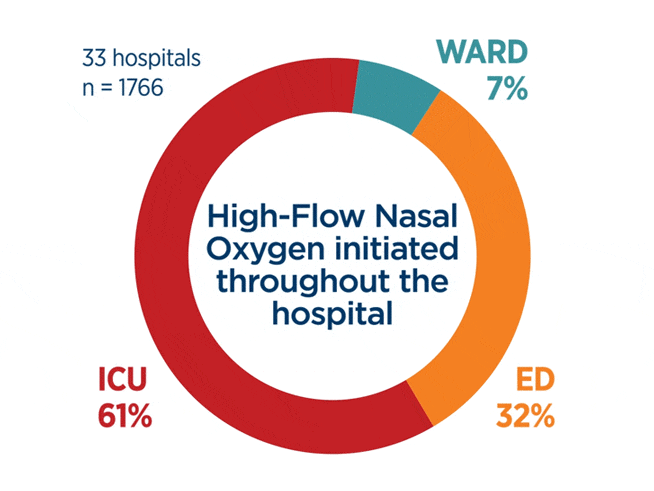
“These results suggest that high flow oxygen can serve as a safe-bridge therapy while the underlying cause of acute respiratory failure is determined, and the most appropriate respiratory support is ultimately implemented.”
Jean-Pierre Frat, MD, PhD; Sylvain Le Pape, MD, PhD & Arnaud W. Thille, MD, PhD (JAMA editorial, 2024)