
Humidification
The benefits of heated, humidified respiratory support is well established within the clinical literature across the care continuum. These benefits are maximized when respiratory gases are delivered as close as possible to body temperature and pressure, in a saturated state. For this reason, F&P recommends that the humidifier be set to Invasive mode whenever delivering support to neonates and infants. A wide range of heated and humidified solutions are available from F&P, including solutions for T-piece resuscitation, noninvasive respiratory support (such as bubble CPAP and NHF) and invasive ventilation.
The benefits of heating and humidifying gases for respiratory support are still relevant during therapeutic hypothermia of the neonate. Guidelines, including a 2021 publication on behalf of the Newborn Brain Society, typically recommend continuing with routine practice of respiratory care during the cooling period, including delivery of heated, humidified gases at normothermic levels.9-11
Neonates and infants are an inherently vulnerable population with developing respiratory systems and unique growth and development challenges. Heating and humidifying the gases provided to these patients has a range of benefits, for their airways, lungs, temperature and energy reserves. For more information on the benefits of heated humidification for neonates and infants, head to our humidification page.
When using the MR850 Humidifier, the invasive mode is recommended for all infant therapies, including noninvasive therapies. Using invasive mode maximizes the benefits of heated humidification by targeting 37 °C and 44 mg/L of absolute humidity and minimizes the work and energy demand placed on the infant.
When using the F&P 950TM Humidifier, the correct mode is automatically chosen based on the circuit you connect to the humidifier.
Our FAQs should not replace any information or guidelines as stated in the product user instructions or local hospital procedures.
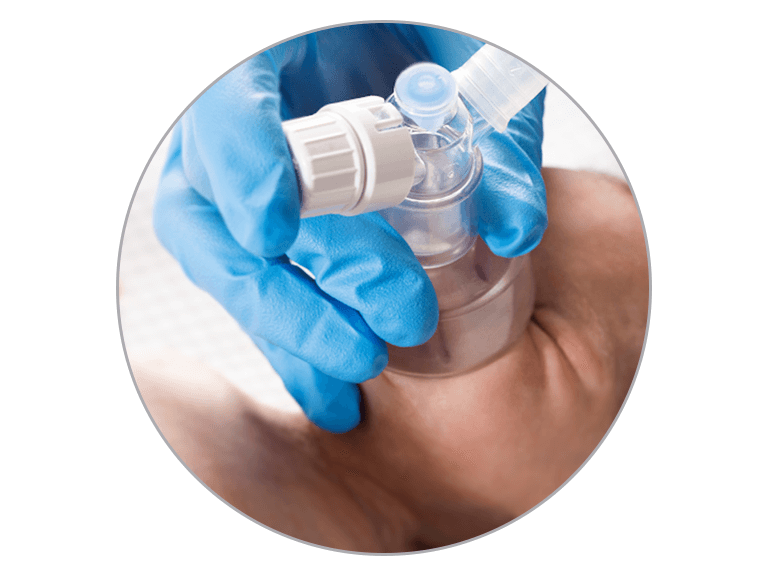
Resuscitation
- NeopuffTM can provide manual inflation breaths (positive pressure ventilation - PPV) and positive pressure support (positive end-expiratory pressure - PEEP) to the patient’s lungs.
- It can be used in any clinical setting where an infant weighing less than 10 kg (22 lb) needs help to breathe. This may include the labor and delivery rooms, operating theaters, birthing units, NICU, special-care nurseries, emergency departments, pediatric ICU and hospital wards.
- Neopuff is only to be used by those trained in infant resuscitation, and with reference to resuscitation guidelines and the Neopuff T-piece resuscitation system user instructions.
- PEEP protects against lung injury and distal airway collapse at the end of expiration, so it improves oxygenation and helps to establish and maintain functional residual capacity.1,2 After an inflation without PEEP, lung volume is lost, the airways collapse, and an infant can be at risk of atelectrauma (damage to the alveoli due to shear stress caused by their repeated collapsing and reopening during the inflations that follow).
- International neonatal resuscitation guidelines suggest that PEEP is especially beneficial during the provision of positive pressure support to preterm infants, and that it should be used if available.3,4
- Rates of approximately 30 to 60 breaths per minute are recommended by most international guidelines. These match the typical respiratory rates of healthy newborns. To match the natural breathing pattern of term and preterm infants, the inspiratory time while delivering PPV should be 1 second or less.4
- The Neopuff T-piece resuscitator can deliver controlled inflations that can be applied to various resuscitation strategies, such as providing longer inflations at the start of resuscitation. However, there is uncertainty around the potential harm or benefit of longer inflations of 1 to 10 seconds, which is why they are used mainly in research studies and are not currently recommended.3,4
- Peak inspiratory pressure (PIP): International guidelines3,4suggest pressures of 20 to 30 cm H2O as initial settings. Pressures should be adjusted up or down according to response. A rising heart rate is a sign that effective ventilation is being delivered.
- PIP is set using the PIP control on the Neopuff T-piece resuscitator.
- Positive end-expiratory pressure (PEEP): When providing positive pressure ventilation (PPV) to newborns, a minimum of 5 to 6 cmH2O is suggested by international guidelines.3,4
- PEEP pressure is set by adjusting the PEEP cap on the T-piece circuit.
- A flow rate of 8 to 10 L/minute is typically used during resuscitation. It is rarely necessary to change the gas flow above the standard rate during resuscitation. If the flow rate is changed, the pressure (PIP and PEEP) settings should be checked again before use.
- The maximum pressure relief valve is a safety feature of the Neopuff which limits the achievable circuit pressure and prevents the accidental delivery of an excessively high PIP. This valve is factory set at a pressure of 40 cmH2O and can be increased if higher pressures are required.
- The classic (900RD010) and ergonomic (RD1300-10) T-piece circuits have a universal connection and can be connected directly to any T-piece resuscitator that meets the gas- powered resuscitator standard ISO 10651-5:2006. The humidified T-piece circuit (900RD110) should only be used with the F&P Neopuff T-piece resuscitator and the F&P MR850 Humidifier.
- F&P resuscitation masks have a 15 mm (0.59”) conical male fitting, and can be connected to common resuscitation devices, including many self-inflating and flow-inflating bags, as well as to non-F&P T-piece circuits which conform to ISO 10651-5:2006.
- Neopuff can be set up in advance of its use. However, it should be checked immediately before every resuscitation to ensure the device is functioning correctly and has the correct PIP, PEEP and safety limit pressure settings. Follow the instructions for use.
- F&P resuscitation masks and T-piece circuits are designed for single-patient use. We recommend you follow hospital protocols regarding infection control and the disposal of consumables.
- The gas supply lines (900RD008 and 900RD009) are reusable products.
- It is recommended that they are checked periodically for cracks, leaks and other damage. If the gas supply lines are not damaged, the maximum use time should be determined by hospital protocols.
- The gas supply lines can be cleaned externally using a detergent-based solution (maximum 2% in water), ensuring the manufacturer’s directions for use of the cleaning agents are followed. If the breathing tube becomes excessively soiled, or soiled internally, it should be replaced.
- The Neopuff T-piece resuscitator should be checked before every use to ensure that the device (including its gas supply line) is functioning correctly. Follow the Neopuff T-piece resuscitator user instructions.
- Dispose of the gas supply lines according to hospital protocols.
Why is mask size important, and how do I select the correct size and type of mask for resuscitation?
- A good mask seal with minimal leak is important to achieve effective ventilation. International resuscitation guidelines recommend that an appropriately sized face mask should cover the mouth and nose but not the eyes and that it should not overlap the chin.
- The Fisher & Paykel Healthcare resuscitation mask range comprises five sizes to fit a range of patients. The masks are designed to be soft and pliable, with a transparent surface that allows breath condensate to be observed. Available diameters: 35 mm (1.37”) (RD803-10), 42 mm (1.65”) (RD804-10), 50 mm (1.97”) (RD805-10), 60 mm (2.36”) (RD806-10) and 72 mm (2.83”) (RD807-10).
- International resuscitation guidelines do not provide recommendations on which type of neonatal resuscitation mask (round vs. anatomical) should be used. Emphasis is instead placed on the correct size, hold and positioning of the mask.
- O’Shea et al. (2016) showed that round masks with an external diameter of 35 mm/1.37” and 45 mm/1.77” were suitable for preterm infants of < 29 weeks PMA (weighing <1000 g/2.2 lb) and 29-33 weeks PMA (weighing 1000-2500 g/2.2-5.5 lb) respectively.5
- You can find out more about mask size and hold when using the F&P Healthcare range of resuscitation masks here.
- No; the use of a nasal cannula to provide ventilatory support with Neopuff is not recommended. Neopuff should only be used with an approved interface, such as a resuscitation mask or endotracheal tube.
- A good seal over the nose and mouth, with minimal leak, is important for establishing effective ventilation. Inadequate seal of the cannula in the nares, and an open mouth, reduces the pressures that can be delivered, which might lead to ineffective ventilation.
- Use of a nasal cannula designed to deliver other respiratory therapies compromises the Neopuff’s ability to provide controlled and consistent pressures, due to increased resistance to flow. In addition, the pressures displayed on the manometer might not accurately reflect the pressures delivered to the infant’s lungs.
- The placement of a filter between the T-piece and resuscitation mask is not advised. F&P Healthcare recommends extreme caution when using filters in unapproved or improvised ways. Resistance to flow, filtration efficiency in different conditions, instrumental dead space, flow dynamics through the system and the potential for gas-trapping are just some of the important safety factors healthcare professionals should consider. Because of these altered parameters, the pressures displayed on the Neopuff manometer may not accurately reflect the pressures delivered to the baby’s lungs.
- Guidelines say that warming strategies, including the use of warmed, humidified inspired gases as a warming aid, may be used to prevent hypothermia in very preterm infants. The latest European Resuscitation Council guidelines further state that the use of warmed humidified respiratory gases should be considered for preterm infants of £ 32 weeks’ gestation who are receiving respiratory support at birth.3
- HHG can be delivered using the Neopuff T-piece resuscitator, MR850 Humidifier, MR290 or MR225 humidification chamber, and the F&P Humidified Resuscitation T-piece Circuit (900RD110). Follow the set-up and operation user instructions.
- As per the MR850 user instructions, it takes up to 30 minutes for the MR850 Humidifier (when in Invasive mode) to reach the set point temperature. Ensure there is gas flowing through the system at all times.
- The published randomized controlled trials by te Pas et al. (2010) and McGrory et al. (2018) describe protocols for providing HHG during stabilization at birth.6,7
- The bench test by Farley et al. (2013) describes a protocol in the laboratory setting.8
The following table summarizes the various protocols used and/or tested for the provision of HHG during resuscitation.
| Study | Volume of water in humidifier chamber | Set-up time | Flow rate used |
| Te Pas AB et al. (2010) | 20 mL | approx. 3 minutes | 8 L/minute |
| McGrory L et al. (2018) | 50 mL | Switched on for im- mediate use | Not specified |
T-piece ventilation can be considered the same as T-piece resuscitation. It refers to respiratory support delivered to neonates and infants via a T-piece resuscitator, such as the F&P Neopuff. T-piece refers to the “T” shape of the patient-end of the circuit that is used with a T-piece resuscitator.
On this circuit, the duckbill port can be used for administering medication, such as surfactant. Refer to your hospital’s protocols for guidance on how the duckbill port is to be used.
Our FAQs should not replace any information or guidelines as stated in the product user instructions or local hospital procedures.
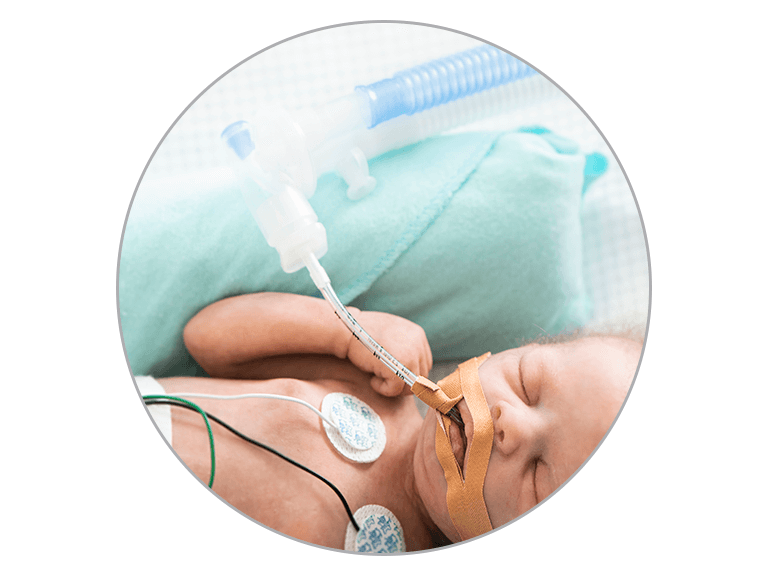
Invasive Ventilation
Condensation can occur in the breathing circuits used with the MR850 Humidifier for several reasons. Some of these are listed below. They can be resolved by making a few small adjustments to the setup.
- Environmental conditions – exposure of the breathing circuits to external heating or cooling, such as the presence of an air conditioning system or a draft from an open window.
- Incorrect setup of the F&P 850 humidification system.
- Loose connections in the circuit setup.
- Use of unapproved circuits or accessories.
Please refer to our brochure, F&P 850 System - Managing Condensation, for more information about how to manage these factors.
- If using the MR850 humidifer, ensure that it is set to Invasive mode.
- When using the F&P 950 humidifier, the device will automatically switch to Neonatal mode when the neonatal circuit is connected.
- For incubators with an air temperature > 34 °C (93°F) use the unheated extension and place the temperature probe just outside the incubator.
- For incubators with an air temperature < 34°C (93°F) and for infant radiant warmers, remove the unheated extension and place the temperature probe in the patient-end probe port located near the patient swivel Y-piece.
- When using an infant warmer or open bedspace, the unheated extension should not be used as it may result in increased levels of condensate, depending on the temperature of the environment around the circuit.
- The unheated extension should not be used during kangaroo care.
Note: Ensure the temperature probe is in the correct position depending on whether the unheated extension is used. This is important as incorrect probe placement may cause inaccurate temperature readings of the circuit due to external heating, such as that from an infant warmer.
* An unheated extension does not come with the F&P 950 neonatal circuit because it is not required when the circuit has built-in Thermadapt™ technology.
- F&P filters (RT016, RT019, RT020) are not intended for use with F&P infant invasive ventilation circuits.
- For pediatric/neonatal ventilators that do not have an inherent expiratory filter and vent to the atmosphere, a disposable filter may be used.
- Ensure the filter has 15 mm connections to facilitate secure connections, which help to minimize dead space and optimize ventilator performance.
- Use only regulatory agency-approved filters. They have been tested in accordance with internationally recognized standards for filtration efficiency against bacteria and viruses.
- Be aware that the filtration efficiency of filters changes with operation. Check the settings on the ventilator periodically and replace filters as per the manufacturer’s specification.
- If you choose to use a filter with our infant invasive ventilation circuits, consider that resistance to flow and filter volume may affect ventilator performance.
F&P humidification systems are suitable for use with a broad range of ventilators and flow sources. Most F&P infant invasive circuits include an adapter kit suitable for a range of ventilator models. Contact your local F&P representative for more information on ventilator-to-circuit compatibility.
Yes; any excess condensate that forms in the circuit can be drained back into the water chamber to avoid unnecessary circuit breaks.
The patient’s own pathogens may be present within the closed circuit. These may be present in condensate which can be drained back to the chamber. However, water vapor generated at the chamber cannot carry pathogens to the patient. Please refer to the information here for clarification around particle size.
Always follow your hospital protocols and the ventilator or nebulizer manufacturer user instructions. Using the appropriate adapter, e.g., RT010 or OPT016, it is possible to introduce medications into the gas path. F&P recommends placing the adapter as close to the patient as possible to reduce potential interference with the humidifier system. Remove the adapter after administering any medication.
Infant circuit kits for the MR850 contain multiple adapters for various clinical scenarios. This includes the RT062 Luerlock adapter, which can be inserted to allow NO delivery, and regular monitoring while using an F&P system. Extra adapters are also available as separate components.
If using the F&P 950 Humidifier, there is a specific kit for delivery of NO (950X08), which contains the required adaptors and a short unheated extension.
If the humidifier is not showing 37 °C, the temperature may have dropped because of changes in ambient conditions (such as drafts or temperature reductions) in order to reduce condensate generation. Too high or too low patient flows can also affect the temperature of the humidifier. Refer to the user instructions for correct set-up and troubleshooting guidelines.
No, it doesn’t matter where the F&P 950 neonatal circuit is positioned. The Thermadapt technology enables independent heating of two zones within the inspiratory limb and allows the F&P 950 System to automatically adapt to the different temperatures in incubators, warmers or the ambient environment. This eliminates the need for an unheated extension and removes the impact of circuit positioning on humidity delivery when this section is positioned relative to the incubator.
For more information, head to the neonatal F&P 950 System webpage.
The duckbill port on infant invasive circuits can be used for suctioning the patient and/or administering medication, such as surfactant. Refer to your hospital’s protocols for guidance on how the duck bill port is to be used.
Our FAQs should not replace any information or guidelines as stated in the product user instructions or local hospital procedures.
CPAP
There are different devices that can be used to deliver CPAP – broadly classified as constant-flow (BCPAP and ventilator CPAP) and variable-flow devices. The body of existing evidence has found BCPAP to be comparable to both ventilator and variable-flow CPAP in regard to improvement of respiratory parameters, such as work of breathing, without differences in therapy failure or the occurrence of adverse events, including nasal trauma and pneumothoraces.12-17
View our Clinical Summary Booklet for more information.
The flow that is delivered through the expiratory circuit is vented to the atmosphere via a tube immersed in water in the bubble generator, which provides a back pressure and generates CPAP.18 The bubbling action of the vented flow enables pressure oscillations that are transmitted down the airways into the lungs.19
Data from randomized controlled trials (RCTs) and clinical guidelines suggests starting pressures at 5 to 8 cm H2O and flow rates of 6 to 10 L/min.20

Several studies, including randomized controlled trials (RCTs) and systematic reviews, show that BCPAP is simple and economical to use and there have been no reports of increased risk of infection from its use.21-24 In general, results from these studies show that the risk of infection is not increased by the system (pressure generator) that is chosen for delivering CPAP therapy.21-24 There have also been no reports of increased risk of infection with the F&P Healthcare bubble generator.
Interfaces are important components in ensuring optimal delivery of CPAP therapy as they create a seal for pressure-based therapies. Interfaces for CPAP therapy have large-bore tubing to lower the resistance to flow. The most common interfaces identified in clinical literature are bi-nasal prongs and nasal masks.25,26
The placement of a filter on the expiratory limb of the BCPAP system is not advised. Resistance to flow, filtration efficiency in different conditions, instrumental dead space, flow dynamics through the system, and the potential for gas-trapping are just some of the important safety factors healthcare professionals should consider. Refer to our COVID-19 Resource Centre for more information.
Our bubble CPAP generator is designed to provide consistent PEEP pressure through an auto-leveling feature. This design moves excess liquid for condensate buildup into a separate chamber from the main pressure-generating section, automatically ensuring that the set pressure does not change with liquid buildup. This overflow container can also be attached to remove the excess water from the system without disrupting therapy delivery.
If delivering BCPAP using the FlexiTrunk™ interface, the interface also contains a moisture-wicking tubing to help manage condensate build up.
Excessive mouth leak can lead to reduced expiratory flow. In the case of end-expiratory resistance CPAP systems, the reduced flow can result in ineffective CPAP therapy. The F&P Healthcare chin strap may be used to reduce any air leak from the mouth.
CPAP, a mode of noninvasive respiratory support, is an accepted alternative to routine intubation and invasive ventilation in preterm infants with respiratory distress syndrome. Refer to our CPAP Therapy page for more information.
CPAP involves application of a single continuous distending pressure throughout the respiratory cycle. Comparatively, PPV refers to the use of two different pressures during the respiratory cycle, consisting of a peak inspiratory pressure (PIP) and a positive end-expiratory pressure (PEEP).
Bubble CPAP is a mode of CPAP therapy in which an underwater seal is created by submerging the expiratory tube in a variable depth of water. Gas exiting the expiratory tube produces bubbles that then generate pressure oscillations.
Bubble CPAP offers additional benefits to the patient which are detailed on our therapy overview page.
CPAP therapy is a well established mode of noninvasive respiratory support for spontaneously breathing patients. Traditionally, CPAP was used invasively, i.e., delivered via an endotracheal tube. However, this is no longer common practice, and CPAP is now delivered noninvasively using nasal interfaces.
Addition of an anti-viral solution to bubble CPAP generators has been documented previously.3 F&P Healthcare has not tested the efficacy of such solutions in terms of their ability to effectively reduce aerosolization of a virus from the bubble CPAP system. However, use of a 0.1% acetic acid solution (vinegar) will have no adverse effects on the material of the bubble CPAP generator. The F&P bubble CPAP generator should always be mounted below the level of the patient.
There are several reasons why you might see pressure sores forming on an infant’s face. Here are some prevention tips:
| Prongs | Mask | |
| Resize | If the prongs are stretching the skin or sliding in and out of the nares easily, the prongs may be sized incorrectly. Use the sizing guide to choose suitably sized prongs. | The mask must not occlude the nostrils or touch the septum nor sit over the lip or eyes. Use the sizing guide to choose a suitable mask. |
| Reposition | Ensure there is at least a 2 mm (0.08”) gap between the prongs and septum. Adjust as necessary. |
General interface tips
Check the nasal tubing height
- If the nasal tubing is slanting up or down and not sitting parallel to the infant’s face, the FlexiTrunk™ interface may need to be adjusted.
- The tubing height can be adjusted by adding or removing foam strips.
Check the nasal tubing length
- The clear tubing should not extend over the patient’s forehead.
- Resize the nasal tubing if necessary.
Consider alternating between the prongs and mask
- Cycling between the nasal prongs and mask “rests” the skin at the seal-contact area and can reduce the risk of irritation. We recommend alternating every four to six hours.
- Hourly checks are recommended to look for signs of redness, pressure sores or irritation that may arise from extended prong or mask use. Frequent checks allow for readjustments to be made before further irritation occurs.
Our FAQs should not replace any information or guidelines as stated in the product user instructions or local hospital procedures.
Nasal High Flow
NHF is a flow-based therapy designed to be an open system. Typically, it consists of a flow source to blend air and oxygen, a heated humidifier to heat and humidify the gas mixture, and a circuit and unsealed interface to deliver humidified gas to the patient.27
The level of pressure generated on NHF is dependent on a range of factors including flow rate and nare occlusion.28 Physiological studies have demonstrated pharyngeal pressure that is similar to, or below, the pressure generated during continuous positive airway pressure (CPAP).29 Data from systematic reviews has found that NHF is associated with no increased risk of adverse events such as barotrauma, compared to CPAP.30
Medical gases are typically cold and extremely dry, and exposing the airway to these conditions has negative implications for both airway reactivity and lung mechanics.31
Adding heat and humidity to respiratory gases prior to inspiration reduces the burden on the airway to condition inspired gas to body temperature and pressure, saturated (BTPS). This has numerous beneficial effects, including optimized mucociliary function,32,33 reduction of respiratory effort,32 and promoting the conservation of energy for growth and development.
These benefits are maximized when inspired air is delivered at BTPS (37 °C, 44 mgH2O/L).32
The necessity of heated humidification during noninvasive respiratory support increases with use of higher flow rates. For this reason, hospital protocols and guidelines suggest that humidification always be used when delivering flow > 2 L/min in infants and children, and > 1 L/ min for neonates.34
Neonates (Newborns up to 1 month)*
Data from neonatal randomized control trials (RCTs) and guidance from clinical experts suggest a starting flow rate between 4 and 6 L/min.35-38
- Post-extubation: A Cochrane Review by Wilkinson et al. found that compared to CPAP, neonates on NHF experienced a significant reduction to rates of nasal trauma, and no difference in rate of treatment failure, re-intubation and other adverse outcomes i.e. pneumothorax and bronchopulmonary dysplasia (BPD).30,35-37
- Alternative to prolonged CPAP: Expert consensus suggests NHF may be a suitable alternative to CPAP for neonates who require prolonged periods of noninvasive respiratory support. At the clinician’s discretion, NHF may be considered as an alternative when the patient is stable on CPAP.30,39
- Primary respiratory support: A recent systematic review concludes NHF (with rescue CPAP available) may be considered for primary respiratory support in preterm infants.35,38,40-43
The weight of clinical evidence for NHF is for infants ≥ 28 weeks GA. Therefore, CPAP remains the gold standard for smaller preterm infants.
Infants and Children (1 month - 12 years)*
The body of evidence suggests that 2 L/kg/min for patients up to 12 kg in weight produces a rapid improvement in respiratory distress, and a reduced need for escalation of therapy. Flow rates for those over 12 kg have been protocolized by the PARIS and FIRST-ABC research groups.44–47
| Weight | Flow Rate |
| Up to 12 kg | 2 L/kg/min |
| 13 - 15 kg | 25 – 30 L/min |
| 16 - 30 kg | 35 L/min |
| 31 - 50 kg | 40 L/min |
| >50 kg | 50 L/min |
For more information, read our pediatric edition of Flow Matters here.
In a large multicenter randomized controlled trial conducted by Franklin et al. (2018) (the PARIS trial), the use of Optiflow Junior to deliver NHF therapy to infants with bronchiolitis early during the hospital admission, resulted in a significant reduction to escalation of care due to treatment failure, compared with standard oxygen therapy.18,23-25
Mayfield et al. (2014)51 observed that an improvement to simple physiological parameters, such as heart rate, respiratory rate, and work of breathing within 60 minutes, is a likely predictor of therapy success, while no improvement is a likely indicator for therapy escalation.51 This approach has been further supported by the body of NHF literature.
Franklin et al. (2018) is the largest NHF RCT to date.44 It was conducted using Fisher & Paykel Healthcare’s NHF system (Airvo 2 and Optiflow Junior) at flow rates of 2 L/kg/min in the emergency department and throughout the hospital. The protocol for this study is freely available and can be accessed here.
Product FAQs
| Flow rate (L/min) | MR850 | F&P 950™ | Airvo™ 2 | Airvo™ 3 | ||
| RT330 | RT331 | 950N40 | 950P40 | 900PT561/900PT562 | ||
| OJR410 XS | 1.1-8 | 1.1-8 | 0.5-10 | 1-10 | N/A | N/A |
| OJR412 S | 1.1-9 | 1.1-9 | 0.5-10 | 1-10 | ||
| OJR414 M | 1.1-10 | 1.1-10 | 0.5-11 | 1-11 | 2-7 | |
| OJR416 L | 1.1-23 | 1.1-23 | 0.5-28 | 1-34 | 2-20 | 2-20 |
| OJR418 XL | 1.1-25 | 1.1-25 | 0.5-31 | 1-36 | 2-25 | 2-25 |
| OJR520 XXL | 1.1-36 | 1.1-45 | 0.5-36 | 1-60 | 10-50 | 10-50 |
*Not all options are available worldwide. Please check with your local F&P representative.
To select an appropriate cannula size, ensure a gap is visible between the prongs and the patient’s nostrils and that the prongs are not causing a seal.
No; Wigglepads can be purchased as a spare if you need to replace them. Wigglepads replacement product codes:
| Part No | Description | Quantity |
| WJR110 | Wigglepad 2 Replacement (XS,S) | 2/pack (20 in each box) |
| WJR112 | Wigglepad 2 Replacement (M, L, XL) | 2/pack (20 in each box) |
| WJR114 | Wigglepad 2 Replacement (XXL) | 20/box |
The F&P WigglewiNGTM can be used in conjunction with the Optiflow Junior 2 and Optiflow Junior 2+ range to stabilize an NG tube to the patient, while maintaining the ability to remove or reposition the interface if necessary.
WigglewiNG product codes:
| Part No | Description | Quantity |
| WJR210 | WigglewiNG (XS,S) | 10/box |
| WJR212 | WigglewiNG (M, L, XL) | 10/box |
| WJR214 | WigglewiNG (XXL) | 10/box |
The Optiflow™ Junior 2 cannula is easy to set up – there’s no extra tape required. Refer to the Optiflow Junior 2 Nasal Cannula Fitting Guide for step-by-step instructions.
*Patient populations defined by the FDA
Our FAQs should not replace any information or guidelines as stated in the product user instructions or local hospital procedures.
References